Interpretation
Alvis offers a variety of services designed to facilitate sure communication in an array of business settings.
For example, our highly experienced team can assist with medical conferences, symposiums, seminars, new product announcements,
press conferences, internal meetings, conventions, negotiations, business discussions, and contracts.
In addition to English, we offer interpretation services for a variety of language pairs.
Please contact us for more information.
Types of interpretation
Simultaneous interpretation
In this approach, interpretation proceeds virtually simultaneously as the speaker speaks. Typically a half-day engagement will require two or more interpreters, while a full day will require three or more. Simultaneous interpretation makes it possible to minimize time loss so that meetings can be conducted in multiple languages.
- Large international conferences, scientific society meetings, symposiums, etc.
- Meetings and lectures where it’s important to keep the proceedings short (due to limited time)
- Engagements where speech needs to be interpreted into three or more languages
- Events with numerous speakers
*In venues that are not equipped for this type of interpretation, it will be necessary to install an interpreters’ booth as well as audio equipment.
Consecutive interpretation
In this approach, the speaker speaks for a short while and then pauses as the interpreter interprets what was just said. Although consecutive interpretation requires more time than simultaneous interpretation (about twice as much time), a higher level of accuracy is possible. In addition, no special interpretation equipment is necessary, making this the least expensive approach.
- Small meetings, lectures, seminars, training sessions, etc.
- Settings where there is enough time to ensure that information is communicated as precisely as possible
- Events with only a few speakers
Whispered interpretation
In this approach, which is typically utilized when only a few individuals require the content to be interpreted into another language, the interpreter offers a whispered, virtually simultaneous interpretation to those individuals alone.
Accompanied interpretation
In this approach, an interpreter accompanies one or more individuals as they tour a plant or company, make a courtesy call, or visit a series of sightseeing destinations.
Languages
English, French, Spanish, Chinese, Korean, German, Russian, Italian, and others (Please contact us for more information about other available languages.)
Fields
Veterinary, Medical, and pharmaceutical sciences, etc.
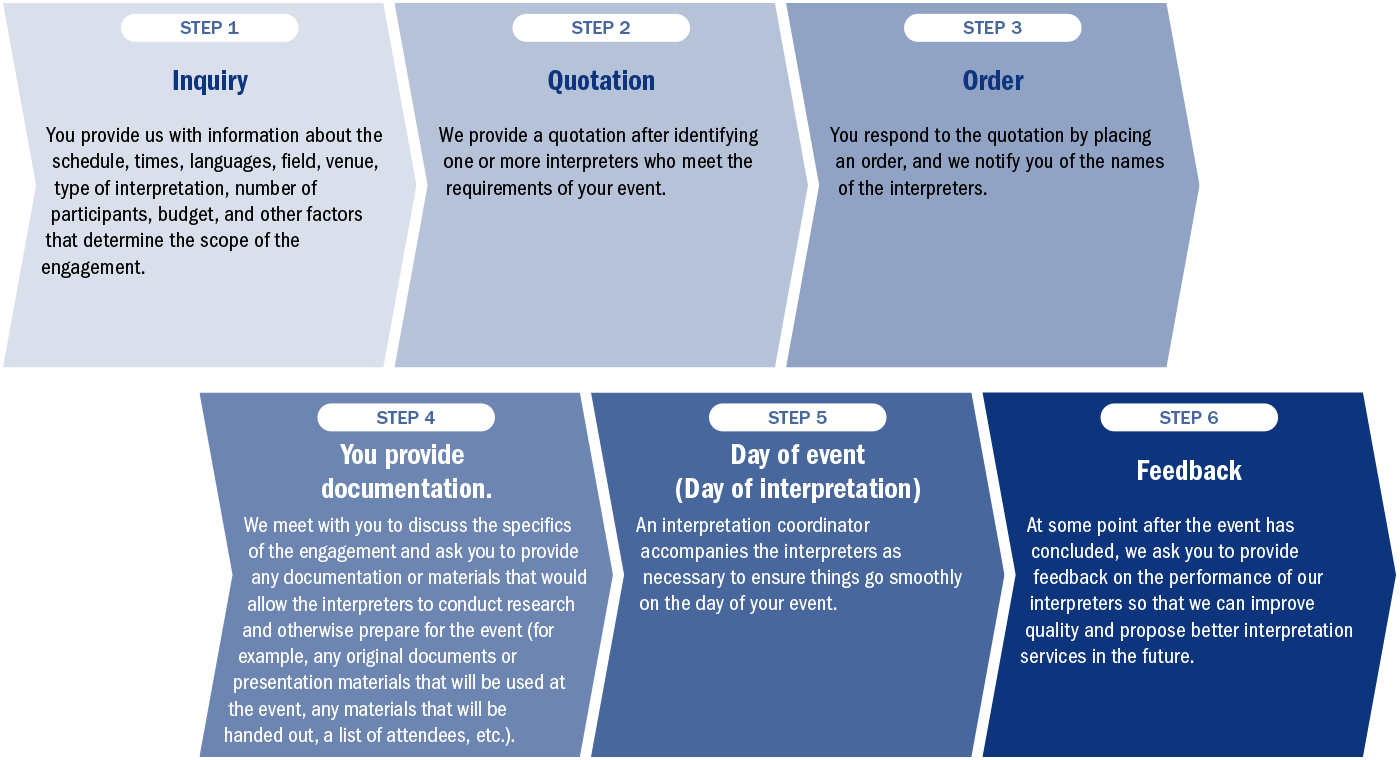
Process
Times
- Half day (up to 3 hours of actual interpretation and 4 hours of on-location commitment)*If more than three hours of interpretation is provided, service will be billed as a full day.
- Full day (8 hours, including a 1-hour break)*After eight hours, additional hours will be billed at an add-on rate.
Fees
Fees vary depending on the type of interpretation, duration of service, field, and number of interpreters required. Please contact us for more information.
*Transportation expenses will be billed separately.
Cancelation policy
Please note that a cancelation fee applies once we have notified you of the names of the interpreter(s) assigned to your event and finalized arrangements. This fee also applies in the event of changes to the time or date of the event.
- Changes made 4 to 5 days before event: 30% of the total quoted amount, including interpretation fees, on-location fees, and other costs
- Changes made 2 to 3 days before event: 50% of the above amount
- Changes made on the day before or day of the event: 100% of the above amount
*A different cancelation policy applies to highly specialized interpretation that includes consulting or other services. Please contact us for more information.